A chemical reaction is the last concept to come to mind when talking about vehicle airbags.
Do you know how an airbag is deployed? Of course, you do! When a vehicle crashes a sensor releases a vehicle’s safety airbags, safely keeping occupants from harm and cushioning the blow.
But, do you know that a chemical reaction is to thank for being able to quickly and safely release a vehicle supplementary restraint system (SRS).
Compressed gas is not used to inflate an airbag, instead, a chemical reaction produces sodium azide or NaN3 to help deploy an airbag.
Why is nitrogen gas used in airbags?
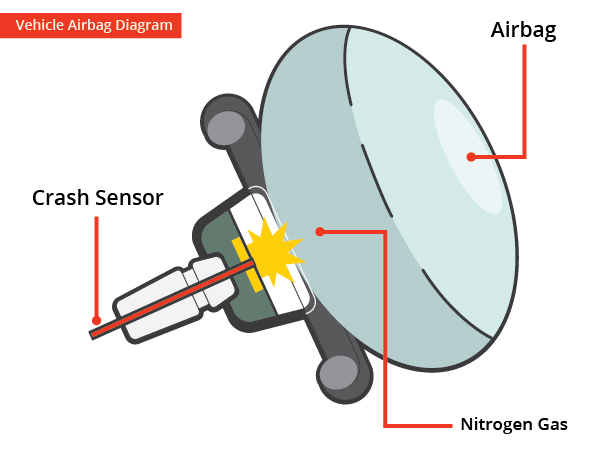
Sensors in the front of a vehicle detect a collision sending an electrical signal to a canister that contains sodium azide detonating a small amount of an igniter compound. The heat from the ignition causes nitrogen gas to generate, fully inflating the airbag in .03 seconds.
Vehicle Airbag Diagram